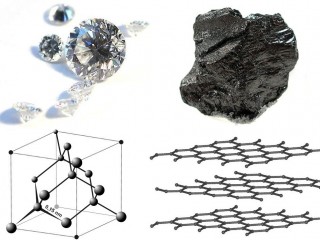
Researchers from North Carolina State University have discovered a new phase of solid carbon, called Q-carbon, which is distinct from the known phases of graphite and diamond. They have also developed a technique for using Q-carbon to make diamond-related structures at room temperature and at ambient atmospheric pressure in air.
http://phys.org/news/2015-11-phase-carbon-diamond-room-temperature.html
The new carbon allotrope has some unusual characteristics: it is ferromagnetic, harder than diamond, and it glows when exposed to low levels of energy. “Q-carbon’s strength and low work-function – its willingness to release electrons – make it very promising for developing new electronic display technologies,” Prof. Narayan explained.
“These objects have a single-crystalline structure, making them stronger than polycrystalline materials,” he added. “And it is all done at room temperature and at ambient atmosphere. So, not only does this allow us to develop new applications, but the process itself is relatively inexpensive.”
http://www.sci-news.com/physics/q-carbon-new-allotrope-carbon-03476.html
Q-carbon is claimed to be an allotrope of carbon announced in 2015. It is described as the third solid phase of carbon, after diamond and graphite. Q-carbon is formed by bringing the temperature of a thin layer of amorphous carbon to 4,000 K (3,700 °C; 6,700 °F) at atmospheric pressure and then rapidly cooling it. Q-carbon is Ferromagnetic, unlike all other known forms of carbon, and Q-carbon appears to be harder than diamond.
https://en.wikipedia.org/wiki/Q-carbon
We report on fundamental discovery of conversion of amorphous carbon into diamond by irradiating amorphous carbon films with nanosecond lasers at room-temperature in air at atmospheric pressure. We can create diamond in the form of nanodiamond (size range <100 nm) and microdiamond (>100 nm). Nanosecond laser pulses are used to melt amorphous diamond like carbon and create a highly under cooled state, from which various forms of diamond can be formed upon cooling. The quenching from the super under cooled state results in nucleation of nanodiamond. It is found that microdiamonds grow out of highly under cooled state of carbon, with nanodiamond acting as seed crystals.
http://scitation.aip.org/content/aip/journal/aplmater/3/10/10.1063/1.4932622